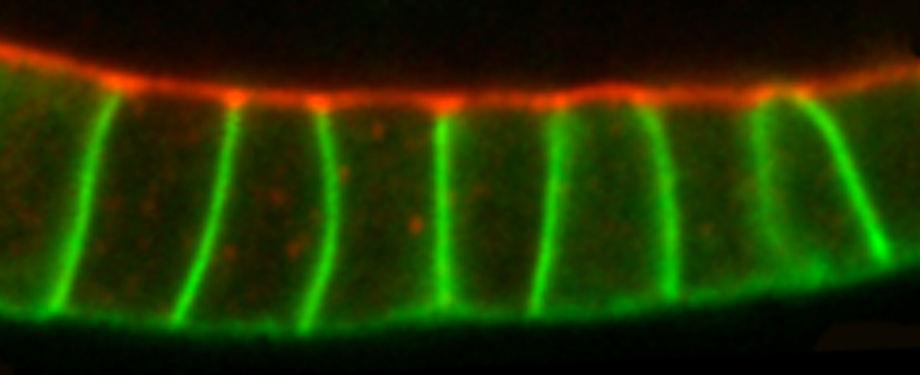
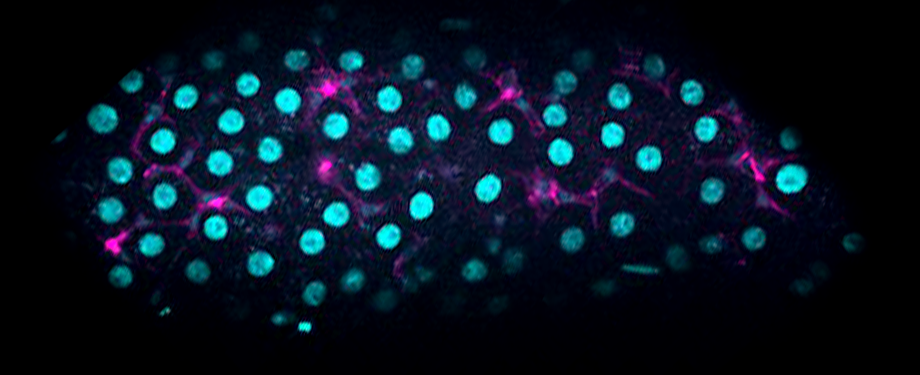
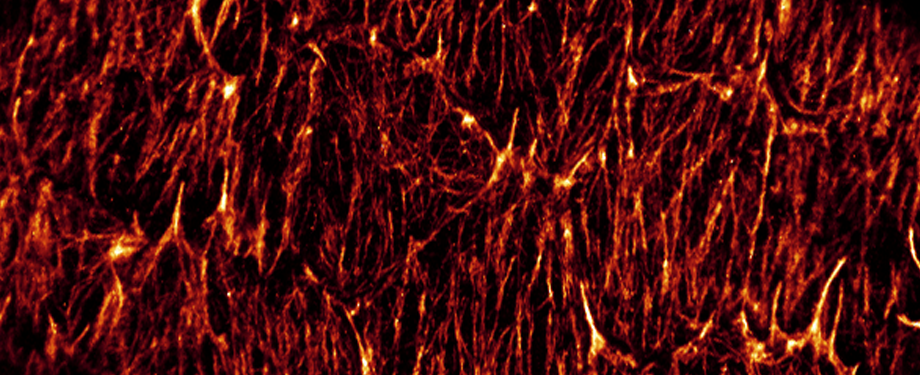
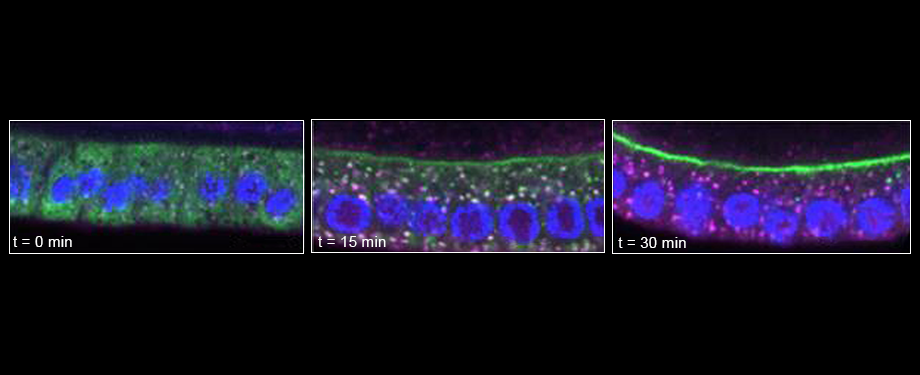
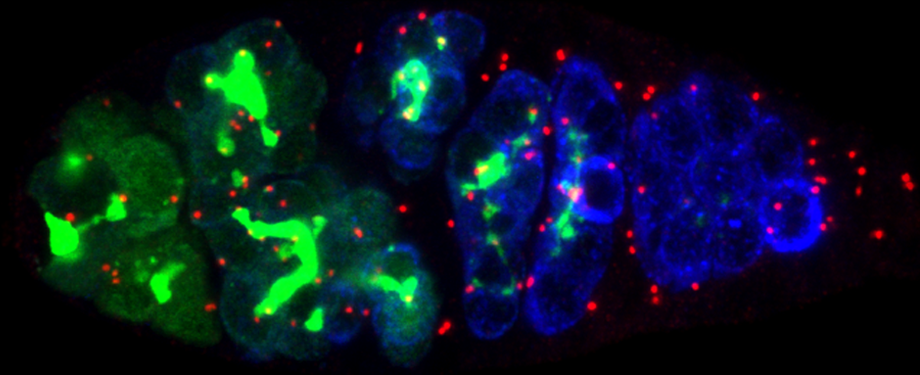
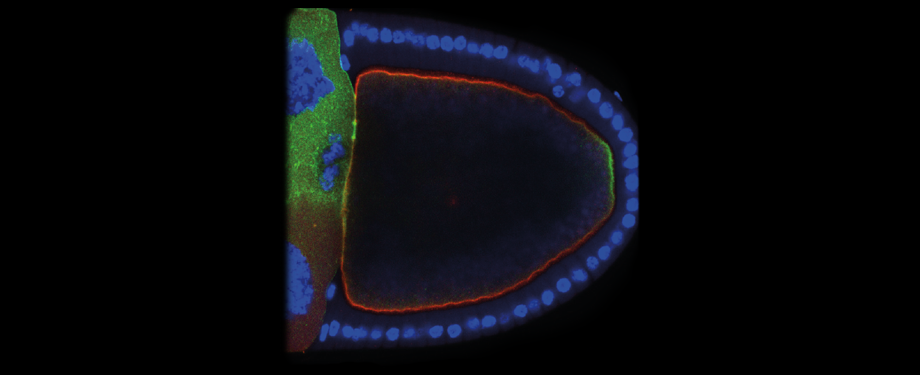
Epithelial cells form sheets that act as barriers which line our organs. To form these sheets the cells must be polarised along their apico-basal axis. Studies in Drosophila have identified a conserved set of polarity proteins responsible for establishing and maintaining epithelial polarity. The polarity proteins can be separated into apical factors (including Crumbs, Stardust, Par-6 and atypical protein kinase C [aPKC]), junctional (Bazooka/Par-3) and basolateral (including Scribble, Discs large and Lethal(2) giant larvae). In addition, Cdc42-GTP acts upstream of Par6/aPKC for apical domain formation. We are using proteomics, live cell imaging and mutant clone analysis to dissect the temporal relationships between these polarity proteins, as well as factors regulating Cdc42 activity.